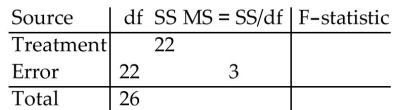
Fill in the missing entries in the partially completed one-way ANOVA table.
-
Definitions:
Isoelectronic
Describes atoms, molecules, or ions that have the same number of electrons and similar electronic structure, but different nuclear charges.
Octet Rule
A chemical rule of thumb that states atoms tend to combine in such a way that they each have eight electrons in their valence shells, giving them the same electron configuration as a noble gas.
Lewis Dot Structure
A diagrammatic method for representing an atom's valence electrons and the chemical bonds between atoms within a molecule.
Helium (He)
A colorless, odorless, tasteless, non-toxic, inert monatomic gas with the atomic number 2, making it the second lightest and second most abundant element in the observable universe.
Q8: A football coach randomly selected ten
Q11: In a random sample of 500 people
Q16: The table below shows the weights,
Q43: The table below shows the number
Q56: Let p1 represent the proportion of men
Q59: When performing a chi-square goodness-of-fit test, how
Q65: An agricultural researcher wishes to compare the
Q66: x = 16, n = 25
Q78: <span class="ql-formula" data-value="\mathrm { x } _
Q92: A poll of 1,068 adult Americans reveals