The image shows bonding for diamond on the left) and graphite on the right) . Both minerals are made of carbon, so why do they have very different properties why is diamond so hard while graphite is so soft) ? 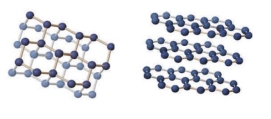
Definitions:
Type 2 Diabetes
A chronic condition that affects the way the body processes blood sugar (glucose), characterized by insulin resistance and elevated glucose levels.
Cigarette-Sponsored Poster
An advertisement material funded by cigarette companies to promote smoking, often seen in magazines, events, or online platforms.
Nonsmoking
The practice of abstaining from the use of tobacco products, associated with improved health outcomes and reduced risk of smoking-related diseases.
Pessimistic Bias
The tendency to overestimate the likelihood of negative events happening in the future.
Q13: Which of the following is NOT a
Q26: A cube of some rock weighs 6
Q40: Which of the following are differences between
Q49: Which letter on the accompanying figure indicates
Q53: On this figure of a continental collision,
Q55: From the list provided below, choose those
Q97: If rock is exposed to enough heat
Q122: Ridge push involves <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5496/.jpg" alt="Ridge push
Q131: Which letter in this classification table indicates
Q183: Which of the following are characteristics typical