The image shows bonding for diamond on the left) and graphite on the right) . Both minerals are made of carbon, so why do they have very different properties why is diamond so hard while graphite is so soft) ? 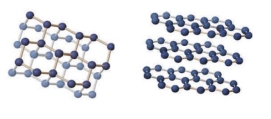
Definitions:
Oil Filter Crusher
A device used to crush oil filters, extracting the used oil and allowing for the metal parts to be recycled.
Refractometer
A tool used to measure the specific gravity of a liquid, such as coolant or battery acid. Light passing through the tool refracts based on the liquid the light passes through. Results are shown on a scale.
Hydrometer
An instrument used to measure the specific gravity of liquids, commonly used in battery maintenance.
Coolant Pressure Tank
A component in a vehicle's cooling system that holds pressurized coolant and helps maintain proper pressure levels within the system.
Q7: An ophiolite is identical to:<br>A) a newly
Q14: Which of the numbered features is a
Q37: Which of the following environments would most
Q42: Which of the following is NOT a
Q47: If a runner races 50 meters in
Q85: Which of the following is NOT a
Q89: Which of the sites shown in this
Q95: Volcanic domes commonly form from all of
Q104: Which of the following situations would result
Q172: Which of the following factors indicates a