Consider a voltaic cell that corresponds to the following reaction: Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s) If this reaction takes place in the electrochemical cell shown in the figure, which of the following statements is incorrect? 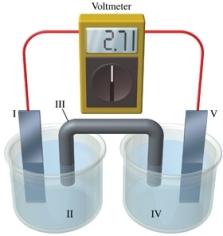
Definitions:
Property Rights
Legal rights over the use, control, and benefits of ownership of property, including intangible and tangible assets.
Living Standards
The level of wealth, comfort, materials, and necessities available to a certain socioeconomic class in a certain geographic area.
Political Stability
The condition of a nation or government that is not likely to experience significant changes, upheaval, or violence.
Property Rights
The legal rights to use, manage, and dispose of assets, including intellectual property and real estate.
Q1: Managers readily use the information generated through
Q3: The figure shows the electrolysis of
Q29: What is the name of the compound
Q37: Marketing research is<br>A)an informational input to decisions.<br>B)a
Q42: Identify the type of solid shown in
Q51: Consider the half-reaction NH<sub>4</sub>+(aq) <span
Q61: What are the parts of a nucleic
Q75: A mercury button battery that is
Q90: Which of the following corresponds to the
Q116: If the initial pressure of a 3.00