Consider a voltaic cell that corresponds to the following reaction: Mg(s) + Sn2+(aq) Mg2+(aq) + Sn(s) If this reaction takes place in the electrochemical cell shown in the figure, which of the following statements is incorrect? 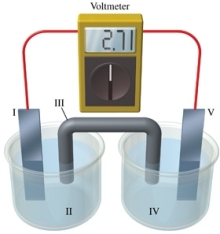
Definitions:
Juan Sánchez Cotán
A Spanish painter renowned for his highly realistic still life paintings, often characterized by a deep exploration of light and form.
Iglesia La Compañía de Jesús
A notable Jesuit church in Quito, Ecuador, renowned for its lavish baroque interior and use of gold leaf.
Cuzco
A city in southeastern Peru, near the Urubamba Valley of the Andes mountain range; it was the historic capital of the Inca Empire.
Retablo
Spanish, “altarpiece.”
Q6: Which of the following is not true
Q6: Which of the following should be most
Q8: Sterling silver is an alloy consisting of
Q13: The information needs for strategy development and
Q15: atoms that have an excess of matter,
Q19: Consider the skeletal equation: Sn<sup>2+</sup>(aq) +
Q35: What mass of sodium nitrate is dissolved
Q49: Consider the melting point of the following
Q57: If the pOH of a borax solution
Q94: What mass of sodium hydroxide is dissolved