Consider a voltaic cell that corresponds to the following reaction: Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s) If this reaction takes place in the electrochemical cell shown in the figure, which of the following statements is incorrect? 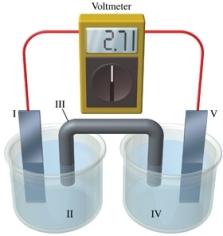
Definitions:
Treaty of Cateau-Cambresis
A series of agreements signed in 1559, ending the conflict between the French Valois and Spanish Habsburgs.
Henry II
A King of England from 1154 to 1189, known for legal reforms that laid the basis for the English common law system.
Albrecht Wallenstein
A prominent military leader and mercenary during the Thirty Years' War, known for his strategy, leadership, and complex allegiances, influencing the outcome of numerous battles.
Protestantism
A branch of Christianity that originated with the Reformation, a movement against what its followers considered to be errors in the Roman Catholic Church.
Q21: Calculate the amount of heat energy,
Q36: Control rods in a nuclear reactor control
Q36: From the vapor pressure curve of propane,
Q47: One of the reasons to conduct research
Q50: Information in existing databases is not useful
Q61: What are the parts of a nucleic
Q83: Which of the following forms of radiation
Q96: If two metals are in contact with
Q105: What are the oxidation numbers of the
Q106: A solid substance has a very high