Consider a voltaic cell that corresponds to the following reaction: Mg(s) + Sn2+(aq) Mg2+(aq) + Sn(s) If this reaction takes place in the electrochemical cell shown in the figure, which of the following statements is incorrect? 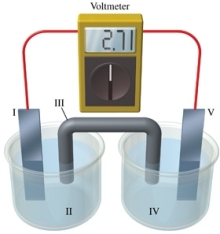
Definitions:
Xenophobia
Fear or hatred of strangers or anything foreign.
Transnationalism
The process of transcending traditional boundaries and operating or extending across national borders, often used in reference to corporations, migrations, or cultural exchanges.
Large States
Political entities that cover a significant geographical area, often characterized by a large population, economic power, and considerable influence.
Macro Level
Refers to a wide-scale approach or perspective, often considering entire systems or societies rather than individual components or behaviors.
Q3: Political acceptability within the firm affects a
Q4: What is the molal concentration of ions
Q19: Marketing research can be used for acquisition
Q27: The reaction that occurs in an
Q29: If the average energy of the products
Q31: A lead-acid battery that is used
Q35: The following ball-and-stick model represents a molecule
Q48: According to collision theory, the increase in
Q65: The critical mass is the amount of
Q75: Which of the following should be most