Consider the reaction N2(g) + 2O2(g) 2NO2(g) .The molecular image represents a mixture of N2(g) and O2(g) just before reaction occurs.What is the limiting reactant, and how much of the excess reactant remains after the reaction is complete? The image contains 3 N2 molecules and 9 O2 molecules. 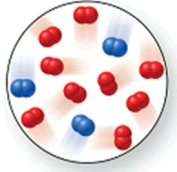
Definitions:
Maturity Value
The total amount that will be paid to the investor at the end of a fixed-term investment, including the principal and any accrued interest.
Promissory Note
A written promise to pay a specific sum of money to a specified person at a definite time or on demand.
Simple Interest
Interest computed exclusively on the principal sum, without adding compounded interest.
Short-Term Investments
Assets that are expected to be converted into cash, sold, or consumed within one year or one business cycle, whichever is longer.
Q12: How much water had to be added
Q20: Which of the following formula/name pairs is
Q24: Which of the following has bond
Q24: In the process of obtaining lead
Q61: Which of the following statements regarding VSEPR
Q65: The kinetic-molecular theory of gases explains the
Q66: The color of visible light with the
Q84: The Lewis symbol for N is: <img
Q94: The name of the oxoanion of nitrogen
Q109: Which element has the abbreviated ground-state electron