Consider the following specific heats of metals. 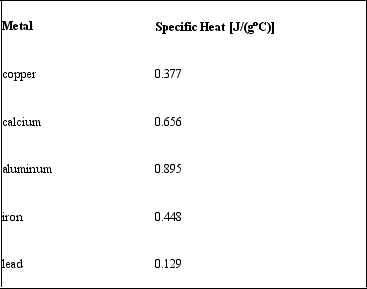 If the same amount of heat is added to 25.0 g of each of these metals, all at the same initial temperature, which metal will have the highest final temperature?
If the same amount of heat is added to 25.0 g of each of these metals, all at the same initial temperature, which metal will have the highest final temperature?
Definitions:
Square Root
A value that, when multiplied by itself, gives the original number, representing the inverse operation of squaring a number.
Simplify Expression
The process of rewriting an expression in a simpler or more compact form without changing its value.
Square Root
A specific value that, when it is multiplied by itself, reproduces the initial figure.
Subtraction
A mathematical operation that represents the operation of removing objects from a collection.
Q32: Calculate the number of moles and
Q44: Identify the class of organic substance for
Q46: Write a balanced equation to represent
Q62: The type of radiation with wavelengths just
Q77: How many molecules are in a sample
Q77: Calculate the number of moles in
Q82: Which element has three completely filled s
Q115: If you have eight bicycle wheels and
Q116: When potassium carbonate, K<sub>2</sub>CO<sub>3</sub>, dissolves in water,
Q116: Elements that have five electrons in the