Consider the Reaction N2(g) + O2(g) 2NO(g)The Molecular Image Represents a Mixture of N2(g) and O2(g)
Consider the reaction N2(g) + O2(g) 2NO(g) .The molecular image represents a mixture of N2(g) and O2(g) just before reaction occurs.What is the limiting reactant, and how much of the excess reactant remains after the reaction is complete? The image contains 2 N2 molecules and 4 O2 molecules. 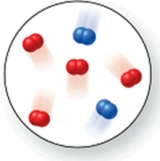
Definitions:
Market Value
The present cost that an asset or service is being traded for in the market.
Book Value
The value of an asset as it appears on the balance sheet, calculated by subtracting any depreciation or amortization from its original cost.
Note Payable
A written promise to pay a specific amount of money, often bearing interest, at a future date.
Market Value
The amount for which something can be sold in a given market at a particular time.
Q3: Rank the following types of electromagnetic radiation
Q5: The molecular-level diagram shows a combination reaction
Q6: Which of the following statements regarding atomic
Q9: Which of the following statements regarding atoms,
Q11: Which of the following statements regarding empirical
Q11: Calculate the amount of heat energy
Q24: Which of the following has bond
Q43: A solution of silver nitrate is mixed
Q52: Which of the following contains a triple
Q56: What is the ground-state electron configuration for