Consider the reaction N2(g) + 2O2(g) 2NO2(g) .The molecular image represents a mixture of N2(g) and O2(g) just before reaction occurs.What is the limiting reactant, and how much of the excess reactant remains after the reaction is complete? The image contains 3 N2 molecules and 9 O2 molecules. 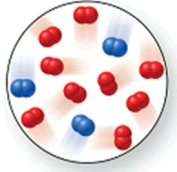
Definitions:
Unsupervised
The process or condition of performing or being without direct oversight or control.
Schizophrenia
A chronic and severe mental disorder affecting how a person thinks, feels, and behaves, characterized by delusions, hallucinations, and other cognitive difficulties, leading to impaired functioning.
On Their Own
Refers to the act of being independent or self-sufficient, without needing help from others.
Group-living Arrangement
A residential living situation where individuals live together and share common spaces, often used for therapeutic communities or supportive living.
Q3: Convert 1.13 atm to torr.<br>A)1.13 x 10<sup>3
Q13: Which combination of formula and name is
Q20: Rank the following elements in order of
Q24: Gaseous nitrogen monoxide reacts with oxygen gas
Q30: If the temperature of a gas at
Q36: When phosphorus reacts with chlorine, phosphorus
Q37: Most molecular compounds remain as molecules when
Q67: Select the pair that has the larger
Q69: A laser used in DVD players has
Q85: One would expect that since CO is