Consider the following specific heats of metals. 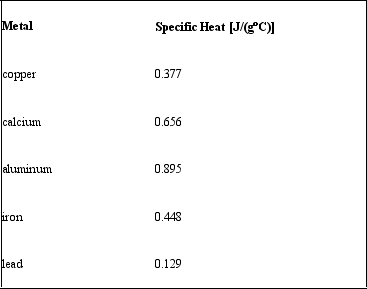 If the same amount of heat is added to 50.0 g of each of these metals, all at the same initial temperature, which metal will have the lowest final temperature?
If the same amount of heat is added to 50.0 g of each of these metals, all at the same initial temperature, which metal will have the lowest final temperature?
Definitions:
Groundwater Chemistry
The study of the chemical characteristics and reactions of groundwater, including dissolved minerals, pollution, and the movement of contaminants.
Ground Surface
The top layer of the earth's crust that we walk on and interact with in our everyday lives.
Volcanic Gas
Gases emitted from a volcano, including water vapor, carbon dioxide, sulfur dioxide, hydrogen sulfide, and other volcanic gases.
Convergent
Pertaining to areas where tectonic plates move towards each other, often resulting in mountain building or subduction.
Q4: For which of the following changes is
Q12: The correctly drawn Lewis formula for SiH<sub>4</sub>
Q35: Which physical characteristic does not apply to
Q63: The overall charge of an atom is
Q64: How much heat energy would be
Q75: Which elements have a partially filled d
Q81: Which of the following statements regarding ion
Q82: Which of the following best describes HCl
Q97: When carbon dioxide is formed from its
Q101: The molecular-level diagram shows a substance which