Use the bond dissociation energies given to estimate the enthalpy change, ΔH°, of the following reaction.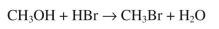
Bond dissociation energies (kcal/mol): C-O, 92; H-Br, 88; O-H, 119; C-Br, 72.
Definitions:
Economic Security
The state of having stable income or other resources to support a standard of living now and in the foreseeable future, protecting individuals and families from poverty and financial uncertainties.
Good Reason
A justified or rational basis for a decision, action, or belief.
Marry
Forming a legally recognized union with a partner.
Personality
The combination of characteristics or qualities that form an individual's distinctive character.
Q1: Given four instructions, how many unique comparisons
Q1: The reaction shown here is one you
Q2: Suppose a program segment consists of a
Q3: When people select an alternative behavior, they
Q4: The dark side of Level Three Leadership
Q10: Draw all the possible addition products formed
Q13: Which of the following structures is properly
Q15: We can cluster leadership skills into these
Q18: Write a recursive mathematical definition for computing
Q32: In which of the following instances would