Boiling point increases as shown in the following series of amines: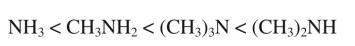
Why is the boiling point of (CH3) 3N lower than that of (CH3) 2NH?
Definitions:
Density-dependent Factor
A factor whose effects on the size or growth of the population vary with the population density, such as disease or food supply.
Population Size
The number of individuals belonging to a specific species that live in a designated area.
Carrying Capacity
The maximum population size of a species that the environment can sustain indefinitely, given the food, habitat, water, and other necessities available in the environment.
Moose Population
A group or number of moose living within a specific area, subject to factors like predation, food availability, and environmental conditions.
Q4: Draw a mechanism for dimerization of the
Q11: What is the product of the following
Q13: d-Sucrose is common table sugar.Its enantiomer, l-sucrose,
Q27: Consider the energy diagram for the multistep
Q29: Which of the following alkenes will be
Q35: Draw the ring flip of the structure
Q40: Provide the IUPAC name for the structure
Q54: Devise a multistep synthesis of the target
Q58: Rank the following compounds in order of
Q59: Sucrose has a specific rotation of