Boiling point increases as shown in the following series of amines: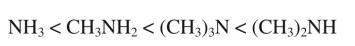
Why is the boiling point of (CH3) 3N lower than that of (CH3) 2NH?
Definitions:
Cognitive Dissonance
A psychological discomfort experienced when holding two or more conflicting cognitions (ideas, beliefs, values, emotional reactions).
Inconsistent
Lacking regularity or uniformity in thought, action, or character.
Controlled
An experiment or situation where variables are manipulated or regulated deliberately to test causality or assess outcomes.
Heider's
Fritz Heider's attribution theory focuses on how people interpret events and relate them to their thinking and behavior.
Q2: Rank the following compounds from least soluble
Q3: Write the lowest-energy electron configuration for a
Q4: Which of these bases can deprotonate acetylene
Q7: Draw a mechanism to account for the
Q11: There are at least two routes to
Q30: How would you make this compound in
Q30: Draw the substitution product that would result
Q38: Which of these structures represent the same
Q41: Which of these ions is aromatic? Assume
Q45: Which of the following Lewis structures shows