For the reaction profile shown below, which statement(s) could be made? 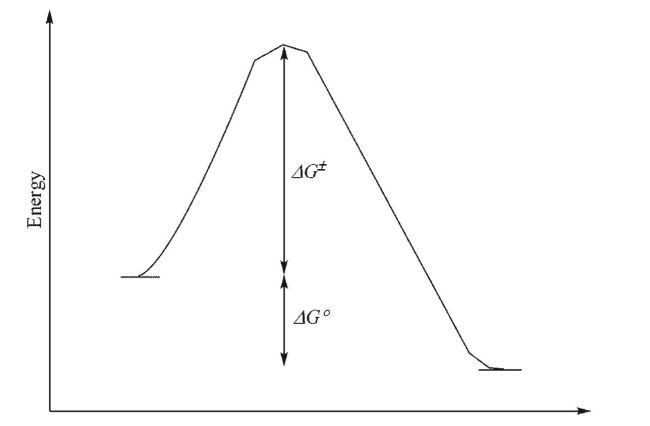
I.The transition state will look more like the starting materials than the products.
II.The reaction is endothermic.
III.The equilibrium constant for the forward reaction will be larger than 1.
IV.The activation barriers for the forward and reverse reactions are equal.
Definitions:
Productivity of Labor
The measurement of the output produced by employees per unit of input, such as per hour of work.
Diminishing Returns
A principle which states that as more units of a variable input (like labor) are added to fixed inputs (like capital), the additional output from each new unit of input will eventually decrease.
Wage Rates
The fixed amount of compensation paid to employees for their labor, typically measured per hour or piece.
Q4: Every organization has a common set of
Q23: Which carbon in the molecule below will
Q24: For the free radical chloriation of 2-methylbutane,
Q31: In the presence of aqueous sulfuric acid,
Q37: Which of the following intermediates has the
Q41: Identify the isoprene units in this structure.
Q46: Which of the following molecules will have
Q56: Which of the following compounds could be
Q63: For the following reactions, indicate what product(s)
Q64: Which of these compounds is the product