What will happen to the rate of the reaction below if the concentration of potassium tert-butoxide is doubled? 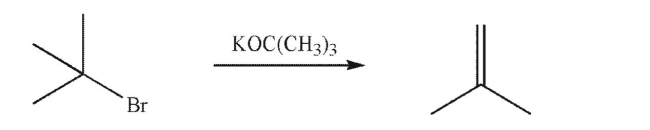
Definitions:
Report Layout View
A feature in Microsoft Access that allows users to design and modify the layout of reports in a more visually intuitive manner.
Format Property
An attribute in software applications that controls the appearance or layout of an object or text.
Short Date
A date format that displays dates in a concise form, typically omitting parts of the date such as the century or leading zeros.
Department Header
An identifier at the top of a document or section that designates its affiliation with a specific department.
Q1: Which of the following is the weakest
Q13: Draw the structures of the following compounds
Q22: Identify the HOMO and the LUMO in
Q31: Draw the mechanism showing the Brønsted acid/base
Q34: Which of the following dichlorocycloalkanes is chiral?
Q44: Draw the π molecular orbitals of allyl
Q49: Draw the energy diagram for the following
Q51: Predict the product of the following reaction.
Q55: Which statement about diastereomers is always true?<br>A)A
Q63: Which of the following molecules could be