For compound A, in acid, it is the hydroxyl oxygen that is protonated to a greater extent than the
carbonyl oxygen. 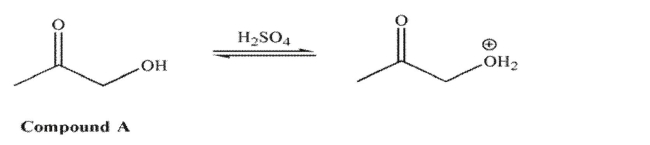 However, for compound B in acid, it is the carbonyl oxygen that is protonated to a greater extent
However, for compound B in acid, it is the carbonyl oxygen that is protonated to a greater extent
than the hydroxyl oxygen. 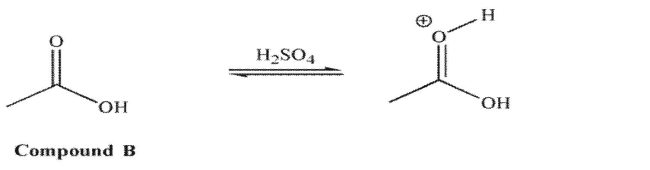 Explain the difference in these results.
Explain the difference in these results.
Definitions:
Marketing And Selling
The process of promoting, selling products or services, and actively engaging customers through various strategies and communication methods.
Social Shopping
An e-commerce method that leverages social media and user contributions to facilitate online shopping experiences.
Shared Via Social Media
Content or information that is distributed and disseminated through social media platforms.
Q1: Draw the intermediates and final product in
Q18: Which statement is true?<br>A)Kinetic resolution is used
Q20: Draw a mechanism for the following transformation.Include
Q27: Which type of sigmatropic rearrangement is involved
Q35: What are the products of this reaction?
Q46: Draw the product of thermal Cope rearrangement
Q51: For each structure, state whether it is
Q73: Which of the following compounds corresponds to
Q81: A random sampling of sixty pitchers from
Q181: A researcher was interested in comparing the