Why is the carbon atom typically the nucleophilic site of an enolate anion? 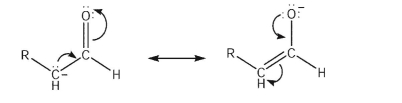
Definitions:
Tax Expenditures
Government revenue losses attributed to tax laws that allow special exclusions, exemptions, or deductions.
Social Policies
Policies designed to address social issues and promote social welfare, often involving governmental intervention to provide services and support in areas like healthcare, education, and housing.
Politically Powerful
Describes individuals or groups that have significant influence over political decisions and policy-making processes.
Government's Social Policies
Strategies and measures developed by a government to address social issues like health, education, housing, and welfare.
Q13: What would be the expected order for
Q21: Which of the following is least likely
Q36: Which of the following structures is
Q36: What is the product of this reaction?<br><img
Q37: Predict the product of the following reaction
Q41: Draw the product of the conrotatory ring-closing
Q43: Draw a mechanism for the transformation shown
Q45: Show the mechanism of the following reaction.<br><img
Q45: Which of the following statements about the
Q78: A Type I error is the mistake