Two chemical solutions, one containing N molecules of chemical A and another containing M molecules of chemical B, are mixed together at time t = 0. The molecules from the two chemicals combine to form another chemical solution containing y (AB) molecules. The rate at which the AB molecules are formed, 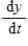 , is called the reaction rate and is jointly proportional to
, is called the reaction rate and is jointly proportional to 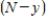 and
and 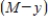 . Thus,
. Thus, 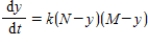 where k is a constant (we assume the temperature of the chemical mixture remains constant during the interaction). Solve this differential equation with the side condition
where k is a constant (we assume the temperature of the chemical mixture remains constant during the interaction). Solve this differential equation with the side condition 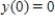 assuming that
assuming that 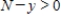 and
and 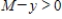
Definitions:
Appeal to Higher Loyalties
A justification for behavior that is based on the claim that the action was in service of a higher principle or greater good, sometimes used to rationalize unethical acts.
Expense Report
A detailed report produced by employees to document and request reimbursement for expenditures made on behalf of the company.
Corporate Social Responsibility
The commitment of a business to contribute to sustainable economic development by working with employees, their families, the local community, and society to improve quality of life in a way that is beneficial for both the business and development.
Legal Constraints
Restrictions imposed by laws and regulations that dictate how a business can operate.
Q1: Which of the following is one of
Q2: A public corporation earns $500,000 in pre-tax
Q6: Sketch the level curves of the function
Q7: Trish Potter is a 40% partner in
Q8: Which of the following deductions is allowed
Q8: Island Tours Inc.earned $80,000 in revenue and
Q29: Find the volume of the solid bounded
Q38: The M'Naghten rule is the foundation for
Q45: The concept of precedent comes from English
Q90: The mass media has significant power in