The volume V (in liters) of a certain mass of gas is related to its pressure P (in millimeters of mercury) and its temperature T (in degrees Kelvin) by the law 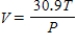
Compute 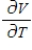 and
and 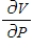 when T = 260 and P = 700.
when T = 260 and P = 700.
Definitions:
Effortful Processing
A form of information processing that requires a considerable amount of attention and conscious effort to encode information.
Geography Course
An academic class focused on the study of the Earth's surfaces, environments, and features, as well as human interaction with the environment.
Various Countries
This term refers to the numerous and diverse nations that exist around the world, each with its own government, culture, and geography.
Atkinson And Shiffrin
Psychologists who proposed the multi-store model of memory, including the sensory register, short-term memory, and long-term memory.
Q4: Min-ju Yang is employed as a salesperson
Q6: Car Co.is selling its land and building
Q13: The total weekly profit (in dollars) realized
Q15: The demand function for Apex women's boots
Q52: Sketch the graphs of the functions f
Q89: Evaluate the definite integral by using the
Q155: Evaluate the following improper integral whenever it
Q186: Find the indefinite integral.<br>Hint: Let <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8255/.jpg"
Q197: Find the approximate change in z when
Q211: Use a double integral to find the