Select the classification for the following reaction. CaCl2·H2O(s) 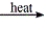 CaCl2(s) + H2O(g)
CaCl2(s) + H2O(g)
Definitions:
Planning Stage
The initial phase where strategies and measures are devised before executing a project or activity.
Produce Message
To create and deliver a communication or information to a receiver or audience.
Message Delivery
The process or method through which a message is conveyed from the sender to the recipient.
Organization
The structured arrangement of parts, people, or processes in a system or entity, or the act of arranging systematically.
Q6: According to the Heisenberg uncertainty principle, if
Q8: Alloying a metal is done to<br>A)make its
Q12: Calculate the rms speed of carbon dioxide
Q24: Select the correct electron configuration for sulfur
Q26: Cold packs, whose temperatures are lowered when
Q40: The electrostatic energy of two charged particles
Q50: Small quantities of hydrogen can be prepared
Q71: Examine the following half-reactions and select the
Q75: A N-14 nucleus is hit by a
Q97: A row of the periodic table is