Multiple Choice
The isotopes of promethium, 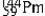 and
and 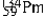 are unstable, and lie on opposite sides of the "line of stability". Which of the following combinations is most likely to represent the type of decay for these isotopes?
are unstable, and lie on opposite sides of the "line of stability". Which of the following combinations is most likely to represent the type of decay for these isotopes?
Definitions:
Related Questions
Q2: Which of the following correctly expresses 0.000007913
Q7: The colorless substance, MgF<sub>2</sub>, is used in
Q8: Calculate the number of oxygen atoms in
Q22: A buffer is prepared by adding 150
Q28: Select the nuclide that completes the following
Q28: For a chemical reaction to be non-spontaneous
Q29: What is the coordination number of cobalt
Q35: Which one of the following is not
Q50: Examine the following half-reactions and select the
Q77: Hydrogen peroxide was catalytically decomposed and 75.3