The temperature at which the following process reaches equilibrium at 1.0 atm is the normal melting point for phosphoric acid. H3PO4(s) ⇄ H3PO4(l)
Use the following thermodynamic information at 298 K to determine this temperature.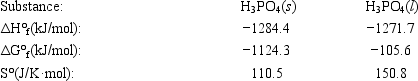
Definitions:
Ohmmeter
A device used to measure electrical resistance in units of ohms.
Serial Link
A Serial Link is a communication interface that transfers data sequentially over a single channel or wire, commonly used in computer and telecommunications systems.
Laptop Computers
Portable personal computers equipped for handling a range of tasks, from document editing to software development.
Truck Chassis Data Connector
An interface point on a truck chassis allowing for the connection and communication with diagnostic or monitoring equipment.
Q11: Calculate the solubility of strontium fluoride, SrF<sub>2</sub>,
Q20: Calculate ΔG° for the reaction of iron(II)
Q35: A 55-kg person exposed to thorium-234 receives
Q37: Examine the following half-reactions and select the
Q39: The higher the pressure of a gas
Q49: All the disintegrations of a sample of
Q61: An elementary reaction is a simple, one-step
Q64: When 20.0 mL of 0.15 M hydrochloric
Q66: The r-process occurs during supernova explosions.
Q70: Aluminum metal reacts with chlorine gas to