The reaction of methane with water to form carbon dioxide and hydrogen is nonspontaneous at 298 K. At what temperature will this system make the transition from nonspontaneous to spontaneous? The data refer to 298 K. CH4(g) + 2H2O(g) ⇄ CO2(g) + 4H2(g) 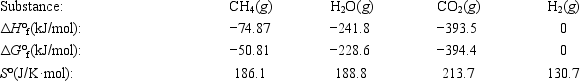
Definitions:
General Adaptation Syndrome
A three-stage response (alarm, resistance, exhaustion) that the body goes through when exposed to chronic stress.
Emotion Work
Emotion work is the process of managing one's own feelings and expressions to fulfill the emotional requirements of a job or personal roles, often involving regulating or changing one's emotional expressions.
Japanese/American Business Meeting
A professional gathering that involves participants from Japanese and American business cultures, requiring an understanding of differing business practices and etiquette.
Too Aloof
A description of someone who is not friendly or forthcoming; cool and distant.
Q12: How many unpaired electrons will there be
Q24: All alcohols are capable of hydrogen bonding.
Q31: The result of (3.8621 × 1.5630) −
Q34: Which one of the following substances will
Q36: Which relationship or statement best describes ΔS°
Q52: Calculate E°<sub>cell</sub> and indicate whether the overall
Q54: Farmers who raise cotton once used arsenic
Q58: Use the following information to calculate the
Q65: In nature, some elements exist as molecules,
Q91: What is the mass-action expression, Q<sub>c</sub>, for