A reaction has ΔG = 10.0 kJ and ΔG° = 15.0 kJ at a temperature of 50°C. Calculate the value of the reaction quotient 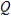 under these conditions.
under these conditions.
Definitions:
Tibialis Anterior
A muscle in the lower leg that dorsiflexes and inverts the foot, playing a key role in walking and running.
Quadriceps Group
A muscle group located in the front of the thigh, responsible for extending the knee and composed of four muscles.
Vastus Lateralis
A large muscle on the side of the thigh, part of the quadriceps group, crucial for extending the knee.
Rectus Femoris
A muscle in the quadriceps that extends the knee and flexes the thigh at the hip.
Q9: Increasing the initial amount of the limiting
Q12: How many unpaired electrons will there be
Q13: Write the mass-action expression, Q<sub>c</sub>, for the
Q34: All bimolecular reactions are second-order reactions.
Q41: The s-process involves a slow succession of
Q52: Calculate E°<sub>cell</sub> and indicate whether the overall
Q59: Which of the following ions could exist
Q70: Which measurement is expressed to 4 significant
Q85: Iron(III) oxide can be reduced by carbon
Q100: Which one of the following combinations of