The temperature at which the following process reaches equilibrium at 1.0 atm is the normal boiling point of hydrogen peroxide. H2O2(l) ⇄ H2O2(g)
Use the following thermodynamic information at 298 K to determine this temperature.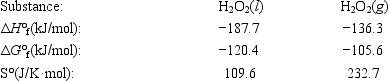
Definitions:
Activity
Any action or series of actions performed by users, systems, or machines, possibly tracked or logged for analysis.
24 Hours
A duration period that consists of a full day, typically used to describe the total hours in a day.
Multiple Calendars
Various calendar systems or schedules that can be used simultaneously for organizing events.
Overlapping Items
Objects or elements that extend over and cover a part of another, often leading to visual clutter or data interpretation issues in various contexts.
Q3: A solution is prepared by mixing 50.0
Q4: Both nylons and proteins are polyamides.
Q29: Consider the reaction CuI(s) ⇄ Cu<sup>+</sup>(aq) +
Q31: Which of the following ligands is most
Q50: An increase in temperature increases the reaction
Q50: Examine the following half-reactions and select the
Q56: The distance between carbon atoms in ethylene
Q57: A reaction has the following rate law:
Q64: What is the pOH of a 0.0085
Q68: What is the value of the equilibrium