The temperature at which the following process reaches equilibrium at 1.0 atm is the normal melting point for phosphoric acid. H3PO4(s) ⇄ H3PO4(l)
Use the following thermodynamic information at 298 K to determine this temperature.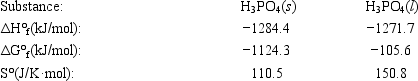
Definitions:
Compliance
The act of adhering to rules, standards, or laws, often within a regulatory, corporate, or legal context.
Servers
Computer systems or software that provide data, resources, or services to other computers, known as clients, over a network.
Warrant
A warrant is an official document issued by a legal or government official authorizing the police or some other body to make an arrest, search premises, or carry out some other action relating to the administration of justice.
Foreign Countries
Nations that are outside the jurisdiction or territory of one's own country, often having different legal, cultural, and political systems.
Q11: Cyclopropane is converted to propene in a
Q34: A characteristic of ligands is that<br>A)they are
Q42: Which of the following has the highest
Q46: Elemental boron can be formed by reaction
Q47: The radioactive isotope tritium decays with a
Q51: Which of the following is a chemical
Q57: What product forms at the anode during
Q66: When metal A is placed in a
Q71: Which of the following acids should be
Q76: Calculate the solubility of silver chromate, Ag<sub>2</sub>CrO<sub>4</sub>,