The reaction of methane with water to form carbon dioxide and hydrogen is nonspontaneous at 298 K. At what temperature will this system make the transition from nonspontaneous to spontaneous? The data refer to 298 K. CH4(g) + 2H2O(g) ⇄ CO2(g) + 4H2(g) 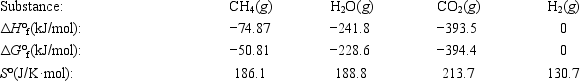
Definitions:
Vasoconstriction
The constriction of the muscular wall of an artery to increase blood pressure.
Dysrhythmia
An abnormal heart rhythm.
Potassium Ions
Positively charged atoms of potassium that play crucial roles in cellular and electrical functions within the body.
Calcium Ions
Positively charged atoms of calcium that play crucial roles in various biological processes, including muscle contraction, nerve function, and blood clotting.
Q6: Ammonia will react with oxygen in the
Q12: The isotope <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8248/.jpg" alt="The isotope
Q24: Which of the following is the empirical
Q31: Which of the following ligands is most
Q43: Aluminum oxide (used as an adsorbent or
Q50: An increase in temperature increases the reaction
Q57: Consider the equilibrium reaction: H<sub>2</sub>(g) + Br<sub>2</sub>(g)
Q58: Use the following information to calculate the
Q81: Which one of the following equations correctly
Q95: Use the following information to calculate the