In the following Lewis structure for ClO3F, chlorine has a formal charge of __________ and an oxidation number of __________. 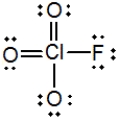
Definitions:
Lee Harvey Oswald
An American former Marine and Marxist who is accused of assassinating President John F. Kennedy on November 22, 1963, in Dallas, Texas.
Assassination
The deliberate killing of a prominent or important person, usually for political or ideological reasons.
Johnson Administration
The presidency of Lyndon B. Johnson in the United States from 1963 to 1969, marked by significant domestic policies such as the Great Society.
Kennedy Disliked Johnson
Refers to the complex personal and professional relationship between President John F. Kennedy and Vice President Lyndon B. Johnson.
Q1: A 4-year-old who had begun drawing in
Q3: The following Bush Administration policy sparked controversy
Q7: Bettelheim believed that autistic symptoms<br>A) are the
Q12: Select the correct reaction type for the
Q19: According to valence bond theory, overlap of
Q19: The following decision governs exculpatory information being
Q34: Which of the following aqueous solutions will
Q49: Consider the reaction 2A + 2B +
Q66: Examine the phase diagram for the substance
Q94: Including cyclic compounds, how many possible isomers