Consider a voltaic cell that corresponds to the following reaction: Cu(s) + 2Ag+(aq) → Cu2+(aq) + Ag(s) If this reaction takes place in the electrochemical cell shown in the figure, which of the following statements is incorrect? 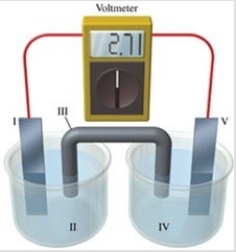
Definitions:
CSR Program
Corporate Social Responsibility program, initiatives by a company to assess and take responsibility for its effects on environmental and social wellbeing.
Order Cycle
The total amount of time that elapses from the time a customer places an order until the time the product is delivered to the customer.
Customer Inquiries
Refers to questions or requests for information made by customers regarding a company's products or services.
Customer Service
The help and counsel furnished by an organization to individuals who acquire or engage with its offerings.
Q3: To which class of compounds does the
Q12: The condensed structural formula for the molecule
Q16: A portion of a DNA strand has
Q17: The trigger voltage of an SCR is
Q29: To which class of compounds does the
Q38: If NaH<sub>2</sub>PO<sub>4</sub> is added to water, what
Q43: A voltaic cell is prepared in which
Q68: Which of the following statements regarding the
Q72: A steel utility pole (made primarily of
Q102: Rank the following compounds in order of