Consider a voltaic cell that corresponds to the following reaction: Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) If this reaction takes place in the electrochemical cell shown in the figure, which of the following statements is incorrect? 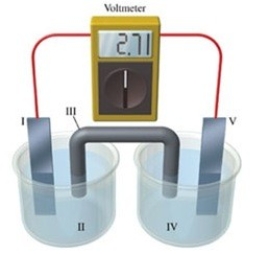
Definitions:
Dividing Mitochondria
Dividing mitochondria refers to the process of mitochondrial fission, where a single mitochondrion divides into two or more separate mitochondria.
Cellular Respiration
The process by which cells convert glucose and oxygen into energy, carbon dioxide, and water, releasing energy to power cell functions.
Photosynthesis
The process by which green plants, algae, and some bacteria transform light energy into chemical energy stored in glucose, using carbon dioxide and releasing oxygen.
Own DNA
Refers to the self-replicating material present in nearly all living organisms as the main constituent of chromosomes, holding genetic information.
Q9: What product is formed when <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8309/.jpg"
Q16: Electronic components are generally more likely to
Q26: Rank the following substances in order of
Q77: Calculate the freezing point of a 2.0
Q90: Calculate the amount of heat energy required
Q90: What is the molal concentration of ions
Q95: The beginning of a strand of mRNA
Q96: Which of the following will not increase
Q101: What type of radioactive decay would be
Q111: The diagram represents the physical state of