Consider a voltaic cell that corresponds to the following reaction: Mg(s) + Sn2+(aq) → Mg2+(aq) + Sn(s) If this reaction takes place in the electrochemical cell shown in the figure, which of the following statements is incorrect? 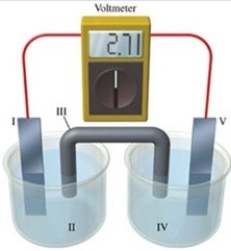
Definitions:
Attitudes
A settled way of thinking or feeling about something, typically reflected in a person's behavior.
Naturalistic Observation
A research method in which subjects are observed in their natural environment without any manipulation by the observer.
Case Studies
Research method involving a detailed and intensive analysis of an individual case or cases, often used in clinical, social, or business research.
Surveys
Research methods involving the collection of data from a sample of individuals through their responses to questions.
Q19: The half-life of technetium-99 is 6.0 hours.
Q25: The maximum on-state output voltage of an
Q41: Consider the reaction: Cu(s)+ 4HNO<sub>3</sub>(aq)→ Cu(NO<sub>3</sub>)<sub>2</sub>(aq)+ 2NO<sub>2</sub>(g)+
Q55: Identify the particle emitted by the nucleus
Q58: Uranium-238 decays by alpha emission to form
Q83: What is the missing symbol in the
Q85: When a piece of copper wire is
Q89: The solubility of potassium chloride is 34.2
Q96: Which of the following formulas represents an
Q111: What is the condensed structural formula for