Consider a voltaic cell that corresponds to the following reaction: Cu(s) + 2Ag+(aq) → Cu2+(aq) + Ag(s) If this reaction takes place in the electrochemical cell shown in the figure, which of the following statements is incorrect? 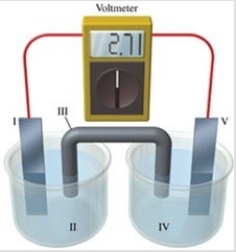
Definitions:
Roman Emblem
A symbol or motif used in ancient Rome to represent a family, legion, or institution, often depicted on shields, coins, and architectural elements.
Houdon
Jean-Antoine Houdon, an 18th-century French sculptor renowned for his portrait busts and statues of philosophers, inventors, and political figures of the Enlightenment.
George Washington
The first President of the United States, a Founding Father, and a leader of the American Revolutionary War.
Baalbek
An ancient city in Lebanon, known for its Roman temples, including the Temple of Bacchus, one of the best-preserved Roman temples in the world.
Q22: Which type of protein structure describes the
Q38: Which of the following should be most
Q40: PWM can be used to create a
Q48: Consider the reaction: 2HgO(s)→ 2Hg(l)+ O<sub>2</sub>(g)Which of
Q66: Write the equilibrium constant expression for the
Q66: How many moles of HCl are contained
Q70: Consider the following reaction carried out in
Q74: The high potential-energy chemical species that is
Q81: What is the name of the straight-chain
Q110: Which of the following corresponds to the