Consider a voltaic cell that corresponds to the following reaction: Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) If this reaction takes place in the electrochemical cell shown in the figure, which of the following statements is incorrect? 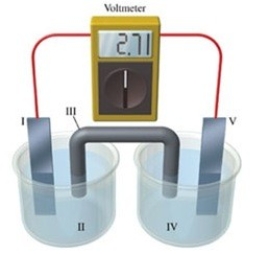
Definitions:
Condenser
A device used to condense a gas or vapor into a liquid, commonly found in air conditioning and refrigeration systems.
Objective
An objective is a specific, measurable, attainable, relevant, and time-bound goal that an individual or organization aims to achieve.
Focus Controls
Refers to mechanisms or systems put in place to maintain or adjust the focus in various equipment or processes.
Objectives
Specific, measurable targets set to achieve goals within a certain timeframe, often defining desired outcomes or achievements.
Q11: An electrolytic cell is an electrochemical cell
Q25: Consider the following reaction: N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)⇌ 2NH<sub>3</sub>(g)+
Q41: Which of the following is the strongest
Q41: The reaction CaCO<sub>3</sub>(s)⇌ CaO(s)+ CO<sub>2</sub>(g)is an example
Q62: Consider the following reaction: Mn(s)+ CuSO<sub>4</sub>(aq)→ MnSO<sub>4</sub>(aq)+
Q76: Arrange the following substances in order of
Q82: What phase transition is occurring between points
Q87: Which of the following changes will increase
Q105: What is the name of the compound
Q105: In which substance does chlorine have an