Consider the reaction and the value of its equilibrium constant: S2Cl2(g) + Cl2(g) ⇌ 2SCl2(g) Keq = 4 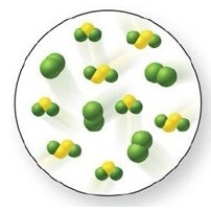 Examine the figure, and determine if the system is at equilibrium. If it is not, in which direction will it proceed to reach equilibrium?
Examine the figure, and determine if the system is at equilibrium. If it is not, in which direction will it proceed to reach equilibrium?
Definitions:
Youth Suicide Rate
A demographic statistic that measures the number of suicides per 100,000 individuals within the youth age group in a given period.
Canada
A country located in the northern part of North America, characterized by vast landscapes, diverse cultures, and two official languages, English and French.
Functionalist Perspective
A theory in sociology that views society as a complex system whose parts work together to promote solidarity and stability.
Conflict Perspective
A theoretical framework in sociology that emphasizes the role of power disparities and competition in shaping social relations and institutions.
Q36: Alpha particles are the least harmful type
Q44: Calculate the number of moles and the
Q46: The total pressure of a mixture of
Q47: Calculate the number of moles and the
Q53: Consider the following reaction at a specific
Q74: The substance HCl(aq)is<br>A)a strong acid.<br>B)a weak acid.<br>C)a
Q87: A lead-acid battery that is used in
Q92: A sample of gas initially occupies 3.35
Q113: Select the solution below that is the
Q116: In January a balloon is taken inside