Consider the reaction and the value of its equilibrium constant: S2Cl2(g) + Cl2(g) ⇌ 2SCl2(g) Keq = 4 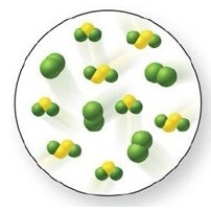 Examine the figure, and determine if the system is at equilibrium. If it is not, in which direction will it proceed to reach equilibrium?
Examine the figure, and determine if the system is at equilibrium. If it is not, in which direction will it proceed to reach equilibrium?
Definitions:
Task Orientation
A focus on completing tasks and achieving specific objectives efficiently.
Cohesion
The force that holds members of a group, society, or material together, promoting unity and consistency.
Internal Communications
The exchange of information and messages within an organization among its members.
External Communications
The process of conveying information and messages from an organization to the outside world, including the public, customers, investors, and other stakeholders.
Q2: Identify the particle emitted by the nucleus
Q14: Which of the following statements regarding the
Q24: Gases are more soluble in water at
Q45: Carbon-14 has a half-life of 5730 yr.
Q65: Why is radon gas dangerous?<br>A)It reacts chemically
Q72: Carbon-14 decays by beta emission to form
Q89: The solubility of potassium chloride is 34.2
Q96: Convert 0.157 atm to mm Hg.<br>A)119 mm
Q103: Rank the following in order of increasing
Q115: Which of the following formulas represents an