The two balloons shown in the figure have the same volume, and are at the same temperature and pressure. Which balloon has the greater number of gas particles, and why? 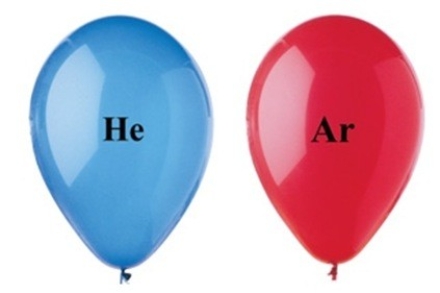
Definitions:
Self-Sacrifice
The act of giving up one's own interests, desires, or well-being for the sake of others or a greater cause.
Moral Principles
Fundamental beliefs about right and wrong that serve as guides for ethical decision making and behavior.
Postconventional Morality
The highest stage in Kohlberg's theory of moral development, where individuals make ethical decisions based on universal principles of justice that transcend societal and personal norms.
Conventional Morality
A stage of moral development where individuals' reasoning is based on societal norms and the desire to uphold laws and rules to maintain social order.
Q11: When completing the orbital diagram for the
Q50: The pictured change of state occurs at
Q63: Which of the following has the highest
Q73: Which of the following statements regarding the
Q96: Consider the following reaction: Cr<sub>2</sub>O<sub>3</sub>(s)+ 3CCl<sub>4</sub>(l)→ 2CrCl<sub>3</sub>(s)+
Q100: How many single bonds are typically formed
Q105: It can be observed experimentally that the
Q115: Calculate the pH of a solution that
Q118: Sodium has a greater first ionization energy
Q129: Small amounts of oxygen gas can be