Consider the reaction N2(g) + 2O2(g) → 2NO2(g) . The molecular image represents a mixture of N2(g) and O2(g) just before reaction occurs. What is the limiting reactant, and how much of the excess reactant remains after the reaction is complete? The image contains 3 N2 molecules and 9 O2 molecules. 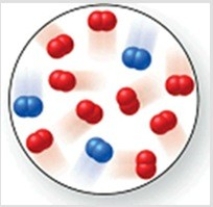
Definitions:
Skin Tenting
A clinical sign often used to assess dehydration that is observed when the skin is pinched and does not immediately return to its normal position.
Urine Output
Urine output is a measure of how much urine is being produced by the kidneys, important for assessing bodily functions and fluid balance.
Blood Pressure
The force of circulating blood on the walls of blood vessels, considered vital for diagnosing various health conditions.
ECV Deficit
Extracellular Volume (ECV) Deficit is a medical condition where there is a reduction in the volume of fluid outside of cells, which can lead to dehydration and electrolyte imbalances.
Q8: Which of the following statements related to
Q16: After the following equation is properly balanced,
Q16: The correct formula for tetraphosphorus hexoxide is:<br>A)PO<sub>6</sub><br>B)P<sub>5</sub>O<sub>6</sub><br>C)P<sub>4</sub>O<sub>7</sub><br>D)P<sub>4</sub>O<sub>6</sub><br>E)P<sub>5</sub>O<sub>7</sub>
Q22: Which choice correctly lists the intermolecular forces
Q66: A steel tank contains gas at a
Q93: Which of the following is a balanced
Q100: Acetic acid is the active ingredient in
Q106: A laser used in DVD players has
Q123: Which of the following statements about isotopes
Q124: A chemical reaction requires 3.50 moles of