Consider the reaction N2(g) + 2O2(g) → 2NO2(g) . The molecular image represents a mixture of N2(g) and O2(g) just before reaction occurs. What is the limiting reactant, and how much of the excess reactant remains after the reaction is complete? The image contains 3 N2 molecules and 9 O2 molecules. 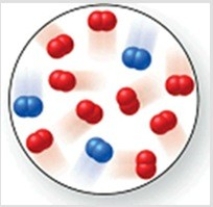
Definitions:
New France
New France was a territory in North America colonized by France, extending from Newfoundland to the Rocky Mountains and from Hudson Bay to the Gulf of Mexico, during the 16th to 18th centuries.
Influential Woman
A woman who possesses the power to affect decisions, opinions, or behavior of others through leadership, creativity, or innovation.
Advancement
The process of promoting or moving forward in terms of development, technology, or career.
Nursing
The profession or practice of providing care for the sick and infirm.
Q22: Which choice correctly lists the intermolecular forces
Q33: Examine the molecular-level diagram. Has a chemical
Q44: Some elements have electron configurations that deviate
Q46: When potassium carbonate, K<sub>2</sub>CO<sub>3</sub>, dissolves in water,
Q100: An energy input of 227 kJ is
Q101: If the theoretical yield for a reaction
Q104: Which of the following formulas for a
Q111: In the Bohr model of the hydrogen
Q121: Which of the following statements is incorrect?<br>A)Warm
Q137: How many moles are in a sample