Which of the molecules in the figure have an empirical formula that is different from their molecular formula? 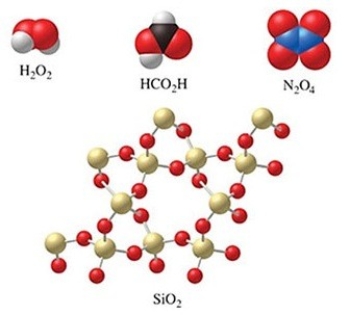
Definitions:
Sample Sizes
The number of observations or units chosen from a larger population for the purpose of statistical analysis.
Weighted Average
An average resulting from the multiplication of each component by a factor reflecting its importance.
Z-score
A numerical metric indicating how a particular value compares to the average of a dataset, expressed by the number of standard deviations it lies from the mean.
P-value
The likelihood of encountering experimental results that are at least as significant as the actually observed results, given the null hypothesis is assumed to be accurate.
Q13: Which particles are found in the atomic
Q35: When an electron moves directly from the
Q35: The correct symbol for the ion formed
Q38: Rank the substances in the figure from
Q63: Sulfur dioxide gas reacts with oxygen gas
Q70: The changes shown in the diagram represent
Q76: Consider the following transformation. Which of the
Q109: The formula for copper(I)sulfide is:<br>A)CuS<br>B)CuS<sub>2</sub><br>C)Cu<sub>2</sub>S<br>D)CuSO<sub>4</sub><br>E)Cu<sub>2</sub>SO<sub>4</sub>
Q115: When phosphorus reacts with chlorine, phosphorus trichloride
Q126: Which ion is expected to be the