Multiple Choice
Rank the substances in the figure from least atoms per mole to most atoms per mole. 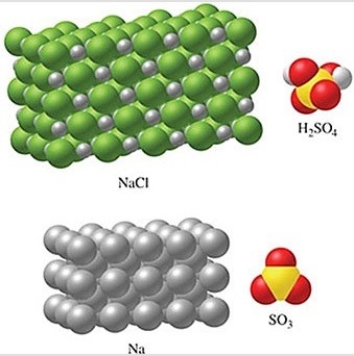
Definitions:
Related Questions
Q30: Consider the reaction Ca(OH)<sub>2</sub>(aq)+ 2HCl(aq)→ CaCl<sub>2</sub>(aq)+ 2H<sub>2</sub>O(l).
Q37: Which element has the abbreviated ground-state electron
Q76: Which of the following formula/name combinations is
Q83: Which of the following contains a triple
Q88: Crystals of red mercury(II)oxide, when heated, form
Q97: Given the following molecular formulas, determine the
Q106: Which set of formulas is correct for
Q112: When 5.0 g CaCl<sub>2</sub> is dissolved in
Q113: Which of the following molecules is a
Q141: For the SO<sub>3</sub> molecule, the Law of