Multiply: 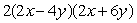
Definitions:
Covalent
A type of chemical bond where atoms share pairs of electrons.
Hydrogen Chloride
A gaseous compound made of hydrogen and chlorine; when dissolved in water, it forms hydrochloric acid.
Lewis Structure
A graphical representation showing the arrangement of valence electrons around atoms within a molecule, indicating bonds and lone pairs.
Lone Pair
A lone pair refers to a pair of valence electrons that are not shared with another atom and are located in the outer shell of an atom, contributing to the molecule's shape.
Q10: A tour operator believes the profit P,
Q12: Graph by using the slope and y-intercept:
Q28: Given that <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8311/.jpg" alt="Given that
Q36: Divide: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8311/.jpg" alt="Divide: A)
Q45: Solve for x: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8311/.jpg" alt="Solve for
Q48: Simplify: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8311/.jpg" alt="Simplify: A)
Q68: Express as a single logarithm. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8311/.jpg"
Q72: Commuting from work to home, a lab
Q84: Simplify: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8311/.jpg" alt="Simplify: A)
Q89: Multiply: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8311/.jpg" alt="Multiply: A)