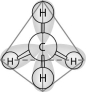
-In the methane molecule shown in the figure above, bonds have formed that include both the s orbital valence electrons of the hydrogen atoms and the p orbital valence electrons of the carbon. The electron orbitals in these bonds are said to be
Definitions:
Null Hypothesis
A statement in hypothesis testing that assumes no effect or no difference between groups or variables.
Type I Error
A Type I Error occurs when a true null hypothesis is incorrectly rejected, also known as a "false positive" finding in research.
Type II Error
An incorrect decision to accept the null hypothesis when it is false.
Punishment
A consequence delivered after an undesirable behavior, intended to reduce the likelihood of that behavior occurring again.
Q2: A climate for service combined with a
Q6: Think of a recent service experience where
Q20: Which of the following is most likely
Q34: The complexity and variety of organic molecules
Q44: The nutritional information on a cereal box
Q45: Large numbers of ribosomes are present in
Q72: What kinds of molecules pass through a
Q81: In the figure above, how many electrons
Q90: Dehydration reactions are used in forming which
Q100: The molecular formula for glucose is C₆H₁₂O₆.