The following questions are based on the reaction A + B ↔ C + D shown in Figure 8.1.
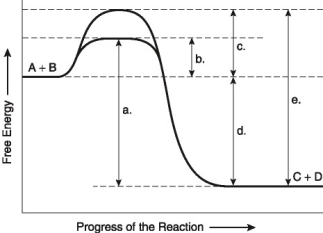
Figure 8.1
-Assume that the reaction in Figure 8.1 has a ΔG of -5.6 kcal/mol. Which of the following would be true?
Definitions:
Maximize Control
The effort to increase one's ability to influence or manage the outcome of events or processes.
Experimentation
The process of conducting controlled tests to explore hypotheses, investigate phenomena, or test theories.
Researchers
Individuals who conduct systematic investigations to establish facts or reach new conclusions in various fields of study.
Correlation Coefficient
A statistical measure that describes the size and direction of a relationship between two variables, ranging from -1 to +1.
Q2: Which of the following most accurately describes
Q3: Increased atmospheric CO₂ concentrations might have what
Q10: Differences among organisms are caused by<br>A) large
Q21: Which of the following statements describes enzyme
Q29: If an adult person has a faulty
Q30: What would be observed by live-cell fluorescence
Q45: Which molecule(s)shown above is (are)ionized in aqueous
Q46: Which functional group shown above is characteristic
Q60: Beta 2 antagonist drugs might also be
Q68: Which chemical group is most likely to