The relation PV = nRT holds for all ideal gases. The additional relation PVγ holds for an adiabatic process. The figure below shows two curves: one is an adiabat and one is an isotherm. Each starts at the same pressure and volume. Which statement is correct? (Note: "∝" means "is proportional to".) 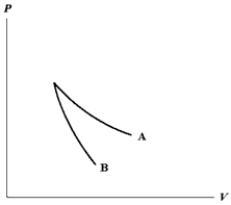
Definitions:
Product Costs
Expenses directly associated with the production of goods or services, including materials, labor, and overhead.
Service Level
A measure of the quality of service provided, including the speed, reliability, and responsiveness of service to meet customer expectations.
Salvage Value
The estimated residual value of an asset at the end of its useful life.
Q1: The path difference between two waves is
Q2: If a supersonic plane is flying at
Q18: What is the mass of air in
Q21: The term <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8245/.jpg" alt="The term
Q31: Two ropes are spliced together as shown.
Q31: An ideal gas is allowed to expand
Q32: Most telephone cables are made of copper
Q51: The total electric flux through a closed
Q70: Refer to Exhibit 16-2. Which of the
Q107: Identical point charges (+30 μC) are placed