Radioactivity: The stability of 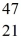 Sc with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known:
Sc with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known: 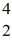 He: 4.002603 u
He: 4.002603 u 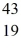 K: 42.960717 u
K: 42.960717 u 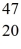 Ca: 46.954543 u
Ca: 46.954543 u 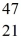 Sc 46.952409 u
Sc 46.952409 u 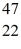 Ti: 46.951764 u The
Ti: 46.951764 u The 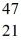 Sc nuclide is
Sc nuclide is
Definitions:
Demonstrative Pronouns
Words used to point out specific things or people, such as "this", "that", "these", and "those".
Declining Enrollment
Describes a situation where the number of individuals registering or enrolling in an institution, such as a school or program, decreases over a period of time.
Microsoft Excel 2013
A version of Microsoft's spreadsheet program that offers new features and improvements over previous versions, providing users with powerful tools for data analysis and visualization.
Topic Sentence
A sentence that expresses the main idea of the paragraph in which it occurs.
Q3: Friction: A person is dragging a packing
Q16: Uncertainty principle using ΔEΔt ≥ ħ/2: A
Q16: Particles: What type of particle is a
Q30: Impulse-momentum: Consider two less-than-desirable options. In the
Q35: Particle in a box: An electron is
Q65: Radioactive decay: Rutherfordium-261 has a half-life of
Q69: Friction: A driver in a <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8274/.jpg"
Q78: Work: You carry a 7.0 kg bag
Q78: Double-slit interference: Light from a 600 nm
Q96: General questions: An object is moving to