Radioactivity: The stability of 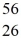 Fe with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known:
Fe with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known: 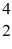 He: 4.002603 u
He: 4.002603 u 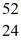 Cr: 51.944768 u
Cr: 51.944768 u 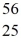 Mn: 55.938907 u
Mn: 55.938907 u 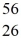 Fe: 55.934939 u
Fe: 55.934939 u 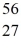 Co: 55.939841 u The
Co: 55.939841 u The 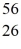 Fe nuclide is
Fe nuclide is
Definitions:
Psychiatric Services
Medical services that focus on diagnosing, treating, and preventing mental health disorders, often involving both medication and psychotherapy.
Transtheoretical Model
A psychological model that outlines stages of change individuals go through in modifying behavior.
Progression
The process of developing or moving forward towards a more advanced state.
Stages
Sequential phases or levels that represent progress or development in a process or a series of events, often used to describe disease progression or life cycles.
Q5: Photoelectric effect: A metal surface has a
Q6: Biomedicine: The radioactive nuclei <sup>60</sup>Co are widely
Q16: Newton's second law: A 60.0-kg person rides
Q43: Hubble's law: A galaxy is observed receding
Q55: Basic kinematics variables: The figure represents the
Q62: Radioactive decay: A hospital patient has been
Q70: Matter waves: In a double slit experiment,
Q89: Dispersion: A beam of light of two
Q120: Multiple-object systems without friction: A series of
Q123: Multiple-object systems without friction: Two objects are