Quantum numbers: If an electron has spin quantum number ms = - 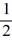 , what is the possible value for the orbital quantum number l of the electron?
, what is the possible value for the orbital quantum number l of the electron?
Definitions:
Financial Reporting
Creating declarations that unveil a company's economic position to its management, investors, and government agencies.
Consolidated Financial Statements
Financial statements that combine the financial information of a parent company and its subsidiaries into one set of statements as if they were a single entity.
Capital Markets
Financial markets for buying and selling equity and debt instruments, serving as a platform for long-term funding for public and private entities.
Q15: Harmonic oscillator: The lowest energy level of
Q15: Bohr atom: A hydrogen atom initially in
Q18: Uncertainty principle using ΔEΔt ≥ ħ/2: The
Q19: Molecular rotation: Estimate the rotational energy (in
Q24: Particles: Which of the following particles are
Q27: Thin films: Light of wavelength 425.0 nm
Q28: Electromagnetic waves: If the magnetic field of
Q31: Radioactive dating: Modern nuclear bomb tests have
Q40: Multielectron atoms: The only VALID electron state
Q45: Lens-maker's formula: A convex-concave thin lens is