Quantity of heat: A 648-g empty iron kettle is put on a stove. How much heat. in joules. must it absorb to raise its temperature from 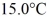 to
to 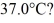 (The specific heat for iron is 113 cal/kg ∙ °C, 1 cal = 4.190 J)
(The specific heat for iron is 113 cal/kg ∙ °C, 1 cal = 4.190 J)
Definitions:
Factorial Research Design
An experimental setup that involves manipulation of two or more independent variables to observe their effect on the dependent variables.
Main Effects
The direct impact of each independent variable on the dependent variable in an experiment.
SSB
Sum of Squares Between groups, a measure used in statistical analysis to quantify the variance among different groups.
SSA
SSA stands for Social Security Administration, a U.S. government agency that administers social security, a social insurance program consisting of retirement, disability, and survivors' benefits.
Q10: Capacitors in combination: When two or more
Q11: First law of thermodynamics: When a fixed
Q19: Capacitors in combination: A 1.0-μF and a
Q28: Molar heat capacities: A quantity of ideal
Q38: Entropy: A brass rod, 75.0 cm long
Q39: Standing waves on a string: A heavy
Q44: Gravitational potential energy: A huge cannon is
Q47: Force on parallel wires: The figure shows
Q50: Resistors in combination: As more resistors are
Q95: Ideal gas law: A sealed container holds